This post is for academic purposes only. Please read the original document if you intend to use them for clinical purposes.
This briefing document summarizes the key information regarding Mpox (formerly Monkeypox) as outlined in the Communicable Disease (CD) alert published in October 2024 by National Centre for Disease Control (NCDC) of Director General of Health Services (DGHS), Government of India. The document highlights the global and Indian scenarios, epidemiology, modes of transmission (including the emergence of a potentially more transmissible and virulent Clade 1b), clinical features, case definitions, surveillance strategies, management principles, contact tracing protocols, preventive measures (including vaccination status), risk assessment, and key public health messages. The resurgence of Mpox globally, particularly the increase in Clade 1b cases and the re-declaration of a Public Health Emergency of International Concern (PHEIC) by WHO in August 2024, underscores the continued public health threat posed by this disease.
Background and Global Situation:
- Mpox is a viral disease of zoonotic origin with Smallpox-like symptoms but less clinical severity. It was first discovered in monkeys in 1958 and identified in humans in the Democratic Republic of the Congo (DRC) in 1970. Historically, cases were primarily confined to African countries. However, in 2022, outbreaks in Europe marked the first time transmission chains lacked known epidemiological links to West or Central Africa.
- WHO initially declared Mpox a PHEIC in July 2022 and re-declared it in August 2024, recognizing the ongoing global threat. Till August 31, 2024, a total of 1,06,310 laboratory-confirmed cases and 234 deaths have been reported to WHO from 123 member states across all 6 WHO regions. The number of monthly reported new cases had increased by 15.6% compared to the previous month (as of August 2024). The African Region (62.3%) and the European Region (13.7%) reported the majority of cases in the preceding month. The top 10 most affected countries globally since January 1, 2022, accounting for 79.9% of reported cases, have been: USA, Brazil, Spain, DRC, France, Colombia, Mexico, UK, Peru, and Germany.
Indian Scenario:
- The first case of Mpox in India in 2022 was reported in Kerala on July 14, 2022.
- A total of 30 laboratory-confirmed cases of Clade II were reported from Kerala (15) and Delhi (15), with the last case reported on March 27, 2024.
- Since September 9, 2024, three new cases had been confirmed with travel history:
- September 9: A 26-year-old male with travel history from China (West African clade-II).
- September 18: A 38-year-old male working abroad with travel history from Dubai (clade 1b).
- September 27: A 29-year-old male with travel history from Dubai (Clade II lineage).
- No secondary cases had been identified in these cases (till the publication of the document).
Agent and Epidemiology:
- The Mpox virus (MPXV) is an enveloped double-stranded DNA virus belonging to the Orthopoxvirus genus.
- There are two distinct genetic clades:
- Clade I (formerly Central African/Congo Basin clade): More virulent and transmissible, showing an upsurge in cases and deaths in the African region since early 2024. The latest outbreak involved a new variant called ‘clade 1b,’ an offshoot of Clade I.
- Clade II (formerly West African clade): The 2022-2023 global outbreak was primarily caused by sub-clade IIb.
- The virus has continued to evolve, with minor genetic changes, gene variants, and a deleted gene observed.
- The natural reservoir is unknown, but certain rodents and non-human primates are naturally susceptible.
- Incubation period: Typically 6-13 days (range: 5-21 days). Individuals are not contagious during this period.
- Period of communicability: 1-2 days before rash onset until all scabs fall off and a fresh layer of skin forms.
- Mode of Transmission:
- Clade 1b: Spreading primarily through household contacts and frequently infects children. Considered capable of spreading faster and killing more people than Clade IIb.
- Clade IIb: Previously spread mainly through sexual contact.
- Human-to-human:
- Large respiratory droplets (requiring prolonged close contact), direct contact with body fluids or lesion material, and indirect contact via contaminated materials (clothing, linens).
- Sexual transmission: Skin-to-skin contact, including sex and oral-genital/oral-cutaneous contact, are risk factors.
- Mother-to-fetus/newborn: Transmission can occur during pregnancy or childbirth.
- Animal-to-human:
- Bite or scratch of infected animals (rodents, non-human primates) or through bushmeat preparation (skinning, eating uncooked meat).
- Pet transmission: Possible through close contact with infected pets.
- Transmission in Children:
- The reason for increased Mpox cases in children is currently unclear.
- Postulated reasons: Adults in endemic areas may have developed immunity through long-term exposure. But children are immunologically naïve.
- Modes of transmission in children:
- Zoonotic Transmission: Spillover from animals.
- Human-to-Human Transmission:Household transmission (significant for children under five).
- Infant transmission (from mothers or close caregivers).
- Person-to-person transmission (estimated 50-60% of cases in children).
- Consumption of Contaminated Animal Products: Direct infection from animals/meat (older children).
- Human-to-human:
Case Definition:
- Suspected case: Any age with a history of travel to affected countries within the last 21 days AND unexplained acute rash AND one or more of the following: swollen lymph nodes, fever, headache, body aches, profound weakness.
- Probable case: Meets the suspected case definition, clinically compatible illness, AND has an epidemiological link to a confirmed case (e.g., face-to-face exposure without PPE, direct physical contact with lesions, contact with contaminated materials).
- Confirmed case: Any case with laboratory confirmation of Mpox virus (PCR and/or sequencing).
Surveillance Strategies:
- Aims: Rapidly identify cases and clusters to prevent further transmission, provide optimal care, identify and manage contacts, protect healthcare workers, and implement effective control measures.
- Key Stakeholders: NACO, IDSP, Points of Entries (PoEs), Hospitals (Derma OPDs, RTI/STI clinics, Antenatal & Pediatric OPDs), and the designated lab network.
- Core Strategies:
- Hospital-based Surveillance: Health facility-based surveillance and testing, especially in relevant OPDs and emergency departments.
- Targeted Surveillance: At NACO-identified intervention sites for MSM and FSW populations, and through Measles surveillance.
- Contact Tracing and Testing: Initiate for symptomatic contacts of probable/confirmed cases.
- Reporting: Immediate reporting of suspected cases to DSU/SSUs and CSU via the specified link. Sample submission to designated laboratories as per guidelines.
- Action upon even one case: Considered an outbreak requiring detailed investigation and contact tracing by all concerned stakeholders.
Clinical Features:
- Usually a self-limiting disease lasting 2-4 weeks. Severe cases are more common in children and are related to virus exposure, health status, and complications.
- Characterized by incubation period, prodrome, and rash:
- Prodrome (0-5 days): May be contagious. Symptoms include fever, lymphadenopathy (typically with fever onset, peri-auricular, axillary, cervical, or inguinal, unilateral or bilateral), headache, muscle aches, exhaustion, chills/sweats, sore throat, and cough.
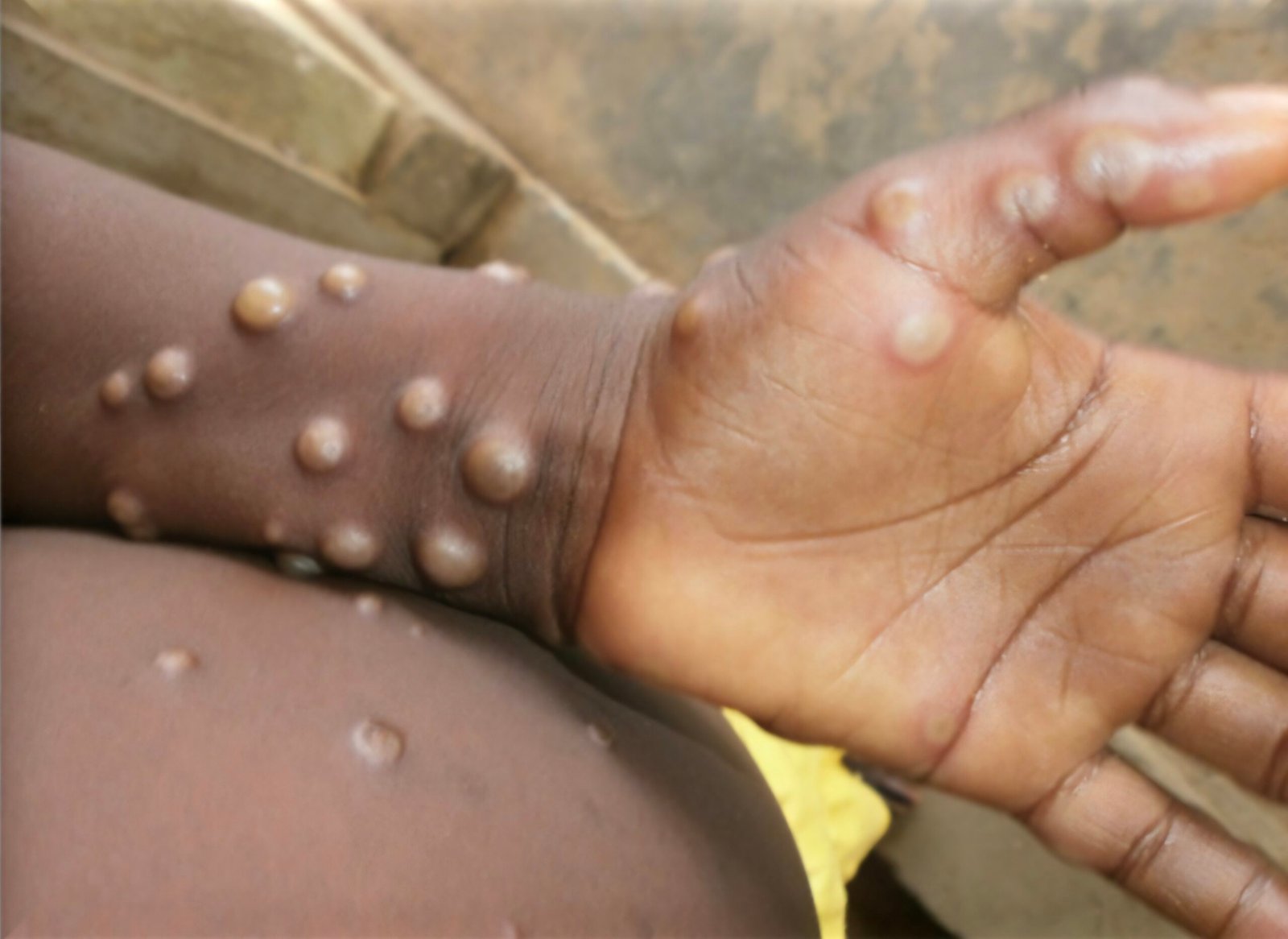
- Skin Involvement (Rash):Typically begins 1-3 days after fever, lasting 2-4 weeks.
- Lesions develop simultaneously and evolve through macular, papular, vesicular, to pustular stages before scabbing and desquamation.
- Deep-seated, well-circumscribed, and often develop umbilication.
- Often painful until healing.
- Stages of rash: Enanthem (oral lesions), macules (face spreading to limbs, palms, soles – centrifugal distribution), papules, vesicles, pustules (classic lesion is vesico-pustular), scabs, desquamation.
- Involvement by area: Face (98%), palms and soles (95%), oral mucous membranes (70%), genitalia (28%), conjunctiva (20%). Limbs and face more affected than the trunk.
- The 2022 European outbreak saw many cases presenting with genital and anal lesions.
- Lesions heal with hyper/hypo-pigmented atrophic scars, patchy alopecia, hypertrophic scarring, and potential facial muscle contractures.
- Predilection for palms and soles is characteristic. Pleomorphic forms of lesions may be present at the same time.
- Skin manifestation can vary based on vaccination status, age, nutritional status, and HIV status.
- High background prevalence of malnutrition, parasitic infections, and other health-compromising conditions can impact prognosis.
- Lesion burden can vary greatly.
- Complications in children: Secondary bacterial infections, severe skin infections, dehydration, pneumonia, encephalitis, ocular complications, and long-term scarring.
- Differential Diagnosis: Varicella, disseminated herpes zoster/simplex, measles, chancroid, secondary syphilis, hand foot & mouth disease, infectious mononucleosis, molluscum contagiosum, and Buffalopox.
Diagnosis:
- Recommended specimen:
- Skin lesion material from multiple sites (swabs of surface/exudate, roofs, crusts).
- Oropharyngeal swab is also recommended if feasible.
- Clinical specimens should be transported to NCDC/NIV Pune/VRDLs through the IDSP network.
Management:
Primarily supportive:
Principles: Patient isolation, protection of skin and mucous membranes, rehydration and nutritional support, symptom alleviation, antibiotics for secondary bacterial infections (if developed), and monitoring/treatment of complications.
- Patient Isolation: Isolation room in hospital/separate room at home, patient to wear a triple-layer mask, skin lesions covered, isolation until all lesions have resolved and a fresh layer of skin has formed.
- Case Fatality Rate: Clade II has a fatality rate of up to 1%, while Clade I has a reported fatality rate of up to 10%. Clade 1b is considered capable of faster spread and higher mortality than Clade IIb.
- Drugs (considered in severe cases, strictly as per physician, NOT self-administered): Tecovirimat, Vaccinia Immune Globulin Intravenous, Cidofovir & Brincidofovir (effective against orthopoxviruses in vitro and animal studies).
Contact Tracing:
- Definition of a contact: Any person with one or more of the following exposures with a probable or confirmed case during the period of communicability: face-to-face exposure without appropriate PPE, direct physical contact (including sexual), contact with contaminated materials.
- Contact identification: Cases should be prompted to identify contacts across various settings.
- Contact monitoring: Home quarantine and active follow-up for contacts of confirmed cases, and self-monitoring for contacts of probable cases for 21 days from the last contact. Clinical/lab evaluation warranted if fever occurs.
- Asymptomatic contacts should not donate blood, cells, tissue, organs, or semen during surveillance.
- Pre-school children may be excluded from group settings.
- Asymptomatic healthcare workers with unprotected exposure should undergo active surveillance for 21 days but do not need work exclusion.
Preventive Measures:
- Raising awareness of risk factors and educating the public is the main strategy.
- General Measures: Avoid contact with materials used by a sick person.
- Isolate infected individuals.
- Practice good hand hygiene.
- Use appropriate PPE when caring for patients.
- Correct containment and disposal of contaminated waste.
- Infection Prevention and Control (IPC) in Healthcare Facilities: Strict adherence to standard and contact isolation precautions, meticulous hand hygiene, appropriate PPE for healthcare workers, environmental cleaning and disinfection with virucidal disinfectants (especially high-touch surfaces), proper waste management, and careful instructions on hand hygiene and PPE for visitors.
- IPC at Home: Patients managed at home must follow all preventive measures in MoHFW guidelines.
- Duration of Isolation: Until all lesions have resolved and a fresh layer of skin has formed. Affected individuals should avoid close contact with immunocompromised persons and pregnant women until all crusts are gone.
Vaccination:
- Three licensed vaccines: modified vaccinia Ankara-BN (MVA-BN/JYNNEOS/Imvamune/Imvanex), LC16-KMB (Japan), and OrthopoxVac (Russian Federation).
- WHO advises vaccination only for individuals at high risk of exposure (certain occupations/circumstances, at-risk travelers) as determined by a healthcare provider. Based on current assessed risks and benefits, mass vaccination had NOT been recommended by WHO for Mpox (till the publication of the document)
- India had also not issued any advisory pertaining to Mpox vaccination (till the publication of the document)
Risk Assessment:
WHO conducted global risk assessment in August 2024 and accordingly:
- High Risk: Eastern DRC and neighboring countries, endemic areas of DRC.
- Moderate Risk: Nigeria and other endemic countries in West, Central, and East Africa, and all other countries in Africa and around the world.
Risk Communication:
- Providing public health advice through targeted channels on disease transmission, symptoms, and preventive measures.
- Community engagement with most at-risk populations, working with healthcare providers (STD/Dermatology clinics), and civil society organizations.
- Communication should be informed by social listening to address misinformation and rumors.
- Health information should avoid stigmatization of any groups (e.g., MSM).
- The IDSP-IHIP community reporting tool can be used to alert the surveillance system.
Next steps:
- Reinforce surveillance activities as outlined in the NCDC alert.
- Enhance awareness campaigns targeting both the general public and healthcare professionals, emphasizing the evolving modes of transmission and the potential for more severe disease with Clade 1b.
- Ensure adequate preparedness in healthcare facilities for diagnosis, isolation, and management of Mpox cases.
- Monitor the global situation and adapt national guidelines as needed based on evolving scientific evidence and WHO recommendations.
- Strengthen collaboration between public health agencies, healthcare providers, and community organizations.
Conclusion:
The document provides a comprehensive overview of the Mpox situation based on the provided NCDC alert. Continued vigilance and adherence to public health guidelines are essential to mitigate the impact of this ongoing public health threat.
Credit for image: WHO